Abstract
Introduction
The JAK1/2 inhibitor ruxolitinib has been approved for the treatment of adults and paediatric patient ≥12 years with steroid refractory graft-versus-host disease (GvHD) based on the REACH-2 and 3 studies. However, real-life studies are needed to validate the results of clinical trials and assess its efficacy in specific organs, particular population groups such as paediatric patients, and possibility to reduce immunosuppression. Moreover, these pivotal studies focused on ruxolitinib after failure to corticosteroids and showed no data about its efficacy after more than one previous line of treatment.
Methods
A descriptive, retrospective, multi-centre study of adult and paediatric patients (<14 years) treated with ruxolitinib for steroid-refractory acute or chronic GvHD from 16 Spanish hospitals belonging to the Spanish Transplantation Group (GETH) between October 2015 and June 2022. The severity of GvHD was evaluated according to the Glucksberg/Magic/Harris criteria for acute GvHD and according to the international consensus of National Institutes of Health (NIH) for chronic GvHD.
Results
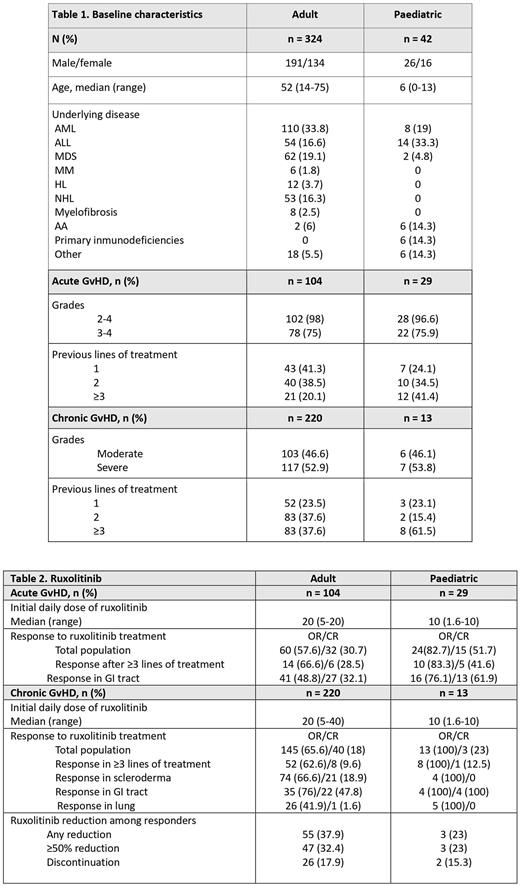
A total of 366 patients were analysed: 324 adults and 42 paediatric patients. Their baseline characteristics and response and toxicity data are described in Tables 1 and 2.
Adult patient group. In acute GvHD, overall response (OR) was observed in 57.6% of patients after a median of 2 weeks of treatment, and 30.7% achieved complete remission (CR). Dose of corticosteroids was reduced in 63.5% of patients. Cytopenias grades ≥3 were observed in 11.3% of patients. Infections included CMV replication (51%), fungal infection (19.2%) and herpes zoster (6.7%). Twelve patients relapsed of their underlying disease. Sixty-six patients died due to: GvHD 17 (25.7%), relapse 4 (6%), infection 36 (54.5%) and other 9 (13.6%). The median overall survival (OS) was 4.1 months, and at 2 years the OS was 28.8% (95%CI: 19.1-39.2). Median follow-up was 2.8 months (range: 0.1-71.6).
In chronic GvHD, the OR was 65.6% and was obtained after a median of 4 weeks of treatment; 18% achieved CR. OR in patients with scleroderma, gastrointestinal tract (GI) or lung involvement was 66.6%, 76% and 42%, respectively. The dose of corticosteroids was reduced in 68.3% and ruxolitinib was tapered in 37.9% of patients. Cytopenias grades ≥3 were observed in 5% of patients. Infections included CMV replication (14%), fungal infection (11.3%) and herpes zoster (5%). Seventeen patients relapsed of their underlying disease. Fifty-three patients died due to: GvHD 13 (24.5%), relapse 4 (7.5%), infection 23 (43.3%) and other 12 (22.6%). The median OS was not reached, and at 2 years the OS was 78.9% (95%CI: 72.5-84). Median follow-up was 26.8 months (range: 0.4-77).
Paediatric patients. In acute GvHD, 82.7% of patients with aGvHD responded after a median of 2 weeks of treatment and 51.7% achieved CR. Corticosteroids were reduced in 79.3% and ruxolitinib in 34.4% of patients. Cytopenias occurred in 3.4% of patients. Infections included CMV replication (34.5%), fungal infection (10.3%) and herpes zoster (17.2%). Four patients relapsed of their underlying disease. Eleven patients died due to: GvHD 3 (27.2%), relapse 2 (18.8%), infection 3 (27.2%) and other 3 (27.2%). The median OS was not reached, and at 2 years the OS was 59.2% (95%CI: 38.2-75.1). Median follow-up was 8.9 months (range: 0.4-59.5).
In chronic GvHD, OR was 100% after a median of 4 weeks of treatment and 23% achieved CR. Corticosteroids and ruxolitinib were reduced in 69.2% and 23% of patients, respectively. Cytopenias occurred in 7.7%. Infections included CMV replication (23.1%), fungal infection (15.4%) and herpes zoster (7.7%). Only 1 patient died due to relapse of the underlying disease; there were 2 relapses in total. The median OS was not reached, and at 2 years the OS was 100%. Median follow-up was 43.1 months (range: 0.9-59.6).
Conclusions
1. Ruxolitinib in the real world setting, showed similar results in terms of responses to clinical trials. In the paediatric population, the data are more favourable: Acute GvHD: 7%; chronic GvHD: 100%.
2. Results seems not be influenced by line of treatment (≥3).
3. By organ, main benetifs in cGvHD were seen in scleroderma (66.6%) and GI (76%) of the affected patients.
4. 5% of adult patients and 79.3% of children with acute GVHD were able to reduce the dose of corticosteroids, while these figures were 68.3% and 69.2% for chronic GvHD.
Disclosures
Escamilla Gomez:Novartis: Research Funding. García Gutiérrez:Roche: Research Funding; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mussetti:GILEAD: Research Funding; JAZZ PHARMACEUTICALS: Consultancy; BMS: Consultancy; TAKEDA: Honoraria. Perez-Simon:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; GILEAD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; JAZZ: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ALEXION: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ABBVIE: Research Funding; PFIZER: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal